Meeting Customer Needs Through Enhanced Product Labelling
For more than 70 years, we have been a leader in IV Therapy by providing parenteral solutions and pharmaceutical injectables that focus on clinical performance and patient safety. Our commitment to patient safety includes providing reliable and progressive products to our customers, which is why we invested in our Canadian manufacturing facility to produce enhanced IV solution labels.
The label enhancements include:
- Improved barcode scanning with inversed white barcode printing
- 2D GS1 Data Matrix with GTIN (01) and additional variable data elements:
- Product lot number (10)
- Product expiration date (17) - Incorporation of Health Canada Plain Language Labelling Regulations
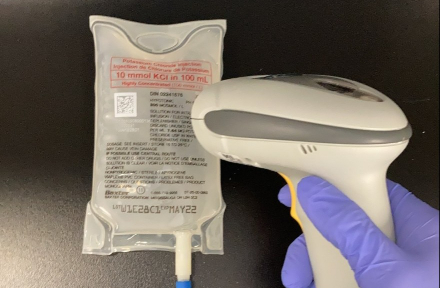
These improvements arm clinicians with vital medication information. Enhanced labelling improves traceability, helps reduce medication errors, and is compatible with electronic medical record systems and automated medication dispensing cabinets. Having barcodes on all packaging levels drives operational efficiency within hospitals, from Receiving and Materials Management to the Pharmacy and clinical staff at the bedside. At the same time, plain language
labelling makes Baxter labels easier to read and understand by all healthcare professionals.
This labelling enhancement initiative aligns with the GS1 Data Matrix Guideline, Institute for Safe Medication Practices (ISMP) Canadian Pharmaceutical Bar-Coding Project, the Health Canada Guidance Document – Plain Language Labelling Regulations for Prescription Drugs, as well as the Health Canada Good Label and Package Practices Guide for Prescription Drugs.
At Baxter Canada, we share the same goals as our customers and clinicians and seize every opportunity to advance patient safety.